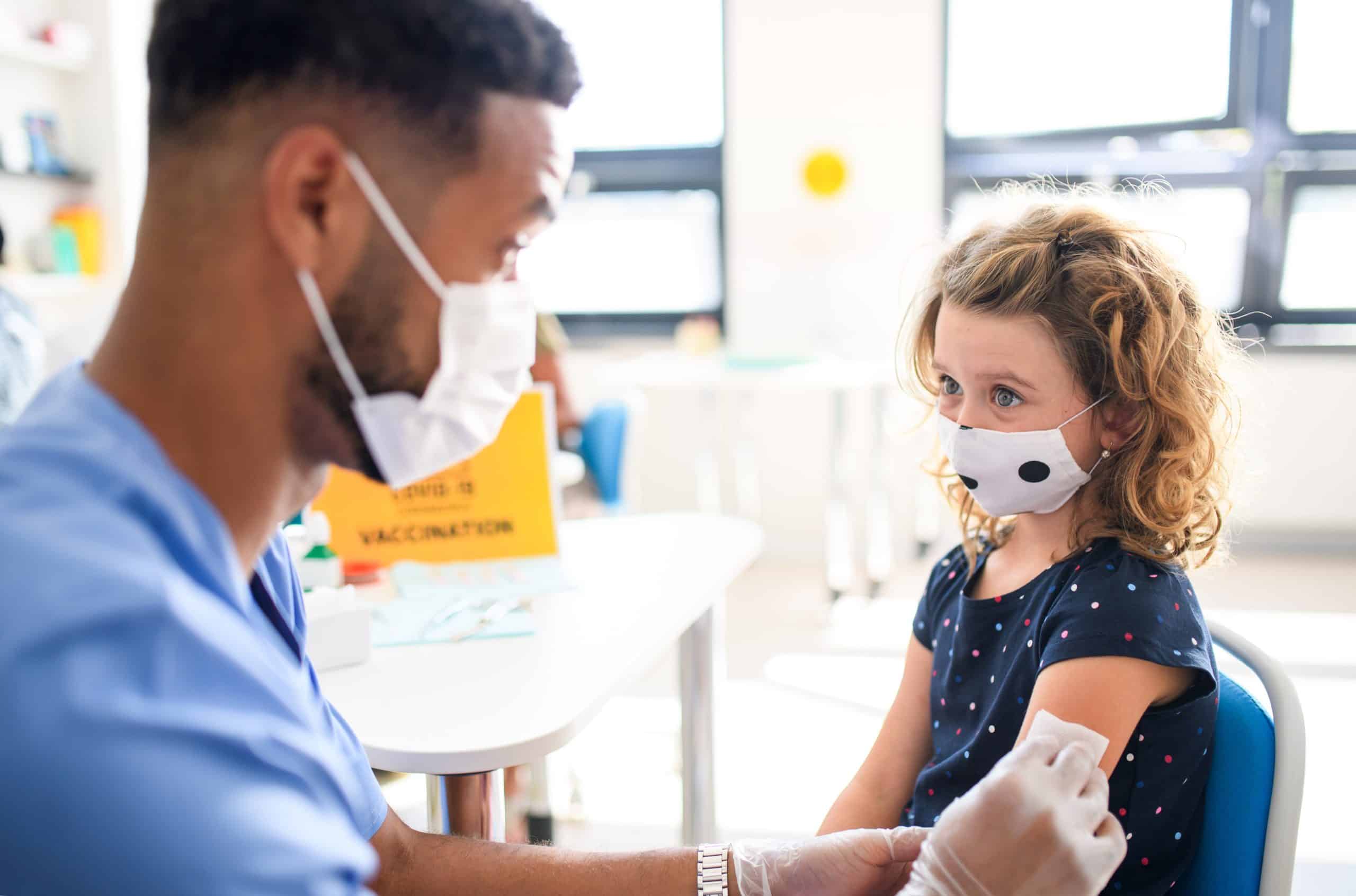
On November 2, 2021, the Centers for Disease Control & Prevention accepted the Advisory Committee on Immunization Practices’ (ACIP) recommendation that children ages 5–11 be vaccinated against COVID-19.
The Pfizer-BioNTech vaccine is now authorized for about 28 million children in the United States in this age group. CDC is allowing providers to begin offering vaccinations as soon as possible.
The dosage for children ages 5-11 will be one-third (1/3) of the approved dosage for adults and teens. CDC Director Rochelle Walensky noted that vaccine effectiveness was nearly 91%. Side effects in clinical trials were similar to those seen in adults (the most common being a sore arm) and were mild.
Children do not need a pediatrician’s authorization or release to get vaccinated at other sites, such as pharmacies or community health centers.
Vaccine distribution started immediately, and will scale up starting on November 8th. Cornerstone Family Healthcare will share more information on the availability of this approved dosage as it becomes available. Check back here for the latest updates on COVID-19 vaccines for children.
Read the full CDC release here.
And here are key insights from Johns Hopkins Bloomberg School of Public Health faculty on why it’s important for kids to be vaccinated.
SOURCE: CDC; Johns Hopkins Bloomberg School of Public Health